There are several mega-trends beginning to interweave that will draw continued attention to the pharmaceutical industry and the safety of the drugs. The first mega-trend results from on-going research and development in the bio-medical sector. For years now, the promise of designer drugs — drugs made for a specific individual — have been dangled in front of consumers. In a post entitled, Healthcare, Technology, and the Supply Chain, I noted that researchers are developing a process that would allow pharmacies to print such designer pills on site [“Printable prescription pills will be safer and faster-acting,” by Darren Quick, Gizmag, 6 June 2010]. Quick reports:
“About two-thirds of all prescriptions are dispensed as solid dosage forms and half of these are compressed tablets. What may surprise many people is that nearly 99.9 percent of most prescription tablets are actually filler. The active ingredient is usually just one thousandth of a pill, so has to be mixed with other ingredients to bulk it out to pill size to make the medicine big enough to pick up and swallow. Now researchers are looking at a fundamental shift in the way pills are produced which promises to create safer and faster-acting medicines – ‘printing’ pills to order.”
Whether or not that occurs, pharmaceutical breakthroughs will continue to be made and new drugs will be in great demand. More specifically, drugs will be required to help meet the demand created by another mega-trend — graying populations. The Economist calls this trend going from the “pyramid to the kite” [“Into the unknown,” 18 November 2010]. The graphic that accompanied the story about Japan’s aging population clearly reveals why they selected that label.
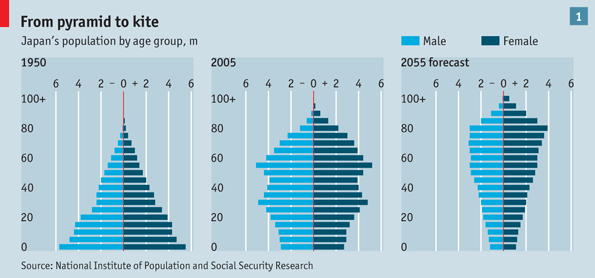
Japan does not face that trend alone. Many developed countries will be moving from the pyramid to the kite. For more on the subject of demographics, read my post entitled Demographics and Economic Growth.
Aging populations and growing pressures to produce ever more exotic drugs have created a third mega-trend — increased healthcare costs. As growing government healthcare costs collide with calls for deficit reductions, politicians and consumers alike are going to look to drug makers for help. Although the pharmaceutical industry successfully rebuffed efforts to permit cheaper, imported drugs into the United States during the recent debates over healthcare, those discussions are likely to be revisited in the years ahead. One of the arguments that drug makers will again raise is that imported drugs are not as safe as those manufactured domestically. As the title of this post implies, I’ll have more to say on the safety of the drug supply chain later.
The fourth mega-trend that is changing the landscape for pharmaceuticals is the rise of emerging and frontier market countries. As reported by the Financial Times, “The result of pressures in established markets is unprecedented [pharmaceutical sector] diversification: nearly all the large pharmaceutical companies, and many of their smaller and speciality peers, are expanding into fast-growing emerging markets, in particular China.” [“Focus shifts to the emerging economies,” by Andrew Jack, 4 May 2010]. Jack discusses the subject of imported drugs mentioned above. He reports, “US regulators – nervous about the globalisation of the industry and especially the drive towards low-cost outsourced manufacturing based abroad – are stepping up factory inspections and warnings.”
The developing world has long had to cope with the specter of fake and substandard drugs. Such drugs are one reason that healthcare in the developing world has remained questionable. In a post entitled Some Updates on Global Healthcare, I discussed how a company in Nigeria was using mobile phone technology to fight counterfeit drugs [“Awash in Fake Drugs, Nigerians Fight Back,” by Will Connors, Wall Street Journal, 12 March 2010]. Connors reports:
“Biofem Pharmaceuticals Ltd., a Nigerian medicine distributor, wanted to arrest a slide in sales after a counterfeit ring targeted its best-selling drug. Sproxil Inc., a start-up founded by a Ghana-born Ph.D. student at Dartmouth, promised to do what Nigerian authorities could not: help companies and consumers detect fake pharmaceuticals. Sproxil’s founder, 28-year old Ashifi Gogo, overcame initial skepticism and a lack of funding to persuade investors to back a technology that offers a quick counterfeit-drug test. The technology could pave the way for wary foreign drug makers to enter the huge African market. … The company has developed technology that allows customers to use their mobile phones to check on newly purchased drugs. Using scratch-off labels and ID numbers, customers can send a code via text message to a database in the U.S. to check whether the medicine they purchased is authentic. Nigeria is Africa’s biggest mobile-phone market, with more than 70 million users.”
India is another country that has a significant problem with fake drugs [“India’s Fake Drugs Are a Real Problem,” by Roger Bate, Wall Street Journal, 19 May 2010]. Bate, a fellow at the American Enterprise Institute, reports:
“In 2009, I looked at five important medicines being sold at 52 different pharmacies in Delhi and Chennai, using covert shoppers. In Dehli, 12% of the pills were substandard, as were 5% in Chennai. About 2% of the pills contained no active ingredient. The others did but it had degraded, probably because the pills had expired and been repackaged with new labels; some may have degraded through poor storage. According to an investigation I just conducted with the Legatum Institute and the International Policy Network, the situation is as bad with at least some of Delhi’s wholesalers. We found that 7% of all tested samples were substandard and 3.6% were likely counterfeit. It’s probable that the drug supply in poorer areas is even more contaminated.”
It might be easy for those living in the West to dismiss this as a local problem. Bate reminds us, however, that “drugs made in India are sold around the world.” As a result, “the country’s substandard drug trade represents a grave public health threat that extends far beyond the subcontinent.” He continues:
“Unless serious steps are taken to improve the quality of the Indian drug supply, the global spread of unsafe pharmaceuticals will persist. It’s no wonder the counterfeit drug trade thrives in India. The profits are enormous. The global trade runs into the tens of billions of dollars. Meanwhile, the penalties for making and selling knock-off medicines are minimal. … The public-health consequences of the counterfeit drug trade are serious. These products, often adulterated with road paint and chalk, look identical to the antibiotics they pretend to be. Thousands of people probably die every year either because they’re poisoned by bad ingredients in counterfeits or because the counterfeit doesn’t treat the victim’s malady. Estimates of substandard drugs reach as high as 30% of all medicines sold in parts of Africa, Asia and Latin America. But the truth is we don’t really how bad the problem has become. These bad drugs don’t just threaten lives. They undermine the legitimacy of the entire Indian medical system, discourage patients from using life-saving pharmaceuticals, and threaten the economic livelihood of legitimate pharmacists and drug makers.”
Bate then puts forth several recommendations for counteracting the global spread of counterfeit drugs from India:
“First, Indian officials need to be more honest about the extent to which bad medicines have contaminated their drug supply. In a 2003 report, India’s Ministry of Health and Family Welfare stated that between 8% and 10% of India’s pharmaceuticals are ‘substandard.’ Yet recent media reports quote unpublished government studies claiming the rate is far below 1%. No one I’ve spoken with believes these figures. India’s leaders must also do more to enforce the anticounterfeit laws already on the books. The penalties for making and distributing bad drugs should be serious and perpetrators should be brought to justice swiftly.””
India’s bureaucracy is famous for its red tape, inefficiency, and corruption. Looking to the government to clean up the mess could mean that the problem languishes for decades. I would suggest that those who stand to benefit the most from cleaning up the pharmaceutical sector (i.e., legitimate pharmaceutical companies) aggressively tackle this challenge. I remind you that it was a start-up company that worked with a pharmaceutical company in Nigeria, not the government, that addressed the problem there. Bate continues:
“New technologies also have a role to play in ensuring the safety of India’s pharmaceuticals. Some drug companies have begun assigning packages—and even individual pills—unique serial numbers. Consumers can check the legitimacy of their medicine by text messaging these serial numbers to a central database and getting a quick and accurate answer.”
Sound familiar? I’m guessing that this is the technology developed in Nigeria by Sproxil Inc. Bate concludes:
“Other quality control solutions involving bar codes also show promise. But any technology will prove effective only if part of a broader political effort to rid India of substandard medicines. Action is urgently required or thousands of annual avoidable deaths around the globe will continue.”
Duane Sword, Vice President, Portable Optical Analysis Unit, Thermo Scientific, agrees with Bate that technology plays a role in ensuring the safety of drug supply chain [“Utilizing Technology to Combat Counterfeit and Substandard Drugs in the Supply Chain,” SupplyChainBrain, 30 November 2010]. He also agrees with me that pharmaceutical companies with the most to gain or lose should aggressively protect the supply chain. Sword writes:
“According to the International Policy Network, more than 700,000 people die each year due to counterfeit malaria and tuberculosis drugs. In emerging markets, counterfeiters exploit vulnerabilities in the supply chain to re-label, repackage, counterfeit and dilute a variety of pharmaceutical products. While it’s more difficult to infiltrate the drug supply chain in developed markets, counterfeiters work around security roadblocks by selling their product directly to consumers over the internet. Pharmaceutical manufacturers are fighting the problem head-on. One large pharmaceutical company has found talcum powder, boric acid, brick dust, highway paint, and even floor wax in counterfeit versions of its medicines. To prevent spurious drugs from threatening patient safety and affecting the bottom line, the company hired former law enforcement officials and customs agents to investigate counterfeit drug manufacturers and distributors worldwide. The company’s global security department has been extremely successful at uncovering criminal activity and involving the appropriate authorities to bring the responsible parties to justice.”
Sword suggests that the pharmaceutical companies not only understand “the different types of supply chain threats they face, but also the tools and technologies available to prevent them.” He continues:
“Whether the issue is a substandard or counterfeit raw material from China entering the legitimate supply chain or counterfeit drugs being sold over the internet, these companies must be prepared to protect their brand, product and customers.”
In order to protect their brand, product, and customers, Sword insists that pharmaceutical companies must harness proven technologies. He continues:
“Technology can help pharmaceutical manufacturers, distributors, wholesalers and others involved in the supply chain differentiate between genuine products and materials and fakes. Whether trying to ensure the integrity of a product or enforcing intellectual property rights, there are technologies that can assist.”
He concludes his article by describing some of the technologies available. He begins with the technology described above that was first developed in Nigeria:
“SMS — One of the newest technologies available to combat drug counterfeiting is SMS, or text messaging. SMS solutions work by affixing a unique code to drug packaging. Once a consumer purchases the drug, he or she reveals the code by scratching it with a coin. The consumer then text messages the unique code to a ‘Mobile Authentication Service’ and receives an immediate response that identifies the drug as legitimate or suspect. Pilot studies have shown this technique to be effective, though it requires that manufacturers alter the drug’s packaging to accept the unique code.”
The most promising aspect of SMS technology is that it works using mobile phones. Mobile phone services have become some of the world’s most pervasive technologies. I noted in a recent post that you can now make a mobile phone call from places as remote as the summit of Mount Everest. Even some of the poorest people on earth have access to mobile phone technology. Sword continues:
“RFID/e-Pedigree — An electronic pedigree is an electronic document that tracks basic data elements of a drug as it travels through the supply chain. Information such as lot number, potency, expiration, manufacturer and other data elements are tracked on an RFID tag from the time a drug is manufactured to final dispensation. The e-pedigree secures the chain of custody, preventing phony transactions and products from getting into or remaining in the legitimate supply chain. Electronic pedigree systems can detect counterfeit and diverted products by analyzing transaction details and suspicious patterns. The downside is that RFID is costly and, like any packaging technology, it does not secure the product itself, making it largely irrelevant in markets where the product is sold without its original packaging.”
Although RFID technology is costly, there are good reasons to believe that the cost of such technology will be decreasing (for more information about RFID technology, see my post entitled Two Views on the Future of RFID Tags). Sword’s next technology is promising but would have limited utility in many developing countries.
“Spectroscopy — Spectroscopy is less well-known, but has been a game-changer in the industry since becoming available in a portable, handheld instrument. This technology can be used in the supply chain to accurately identify chemicals without direct contact with the substance, through sealed glass, plastic bottles, bags and blister packs at ports of inspection, loading docks, points of sale and manufacturing plants. It has been used by a majority of the largest pharmaceutical manufacturers, as well as regulatory bodies around the world.”
Sword, like Bate, believes that governments can also play an important role in protecting the drug supply chain. He continues:
“Governments around the world are also utilizing new technologies, including those highlighted above, to prevent counterfeit and substandard drugs. Nigeria has been a pioneer in the fight against counterfeit drugs, recently bringing counterfeit pharmaceutical levels down from 42 percent to 16 percent thanks to an aggressive policy of intercepting shipments and pursuing counterfeiters. This success can be attributed in part to the use of two of the technologies outlined above, which have allowed the Nigerian National Agency for Food and Drug Administration and Control (NAFDAC) to raise the quality and inspection standards in Nigeria while giving it a greater ability to identify and remove counterfeit drugs from the supply chain.”
Sword notes that “threats in the pharmaceutical supply chain aren’t new, nor are they going to disappear anytime soon.” Wherever there has been a profit to be made, snake oil salesmen have been around to sell counterfeit goods to an unsuspecting public. Fortunately, as Sword notes, “there are steps that companies can take to mitigate risk and ensure patient safety.” He concludes:
“New technology has moved the battle against counterfeit and substandard drugs from the laboratory into the field, enabling pharmaceutical companies, government entities and patients to take control of drug quality.”
In the years ahead, I’m sure that there will be other breakthroughs that will help ensure the public’s safety. With billions of dollars on the line, drug companies would be wise to band together as well as work with governments to help secure the safety of the pharmaceutical supply chain.




